What is Hydroxyapatite: Unveiling the Secret of Bone Composition
|
|
Time to read 9 min
|
|
Time to read 9 min
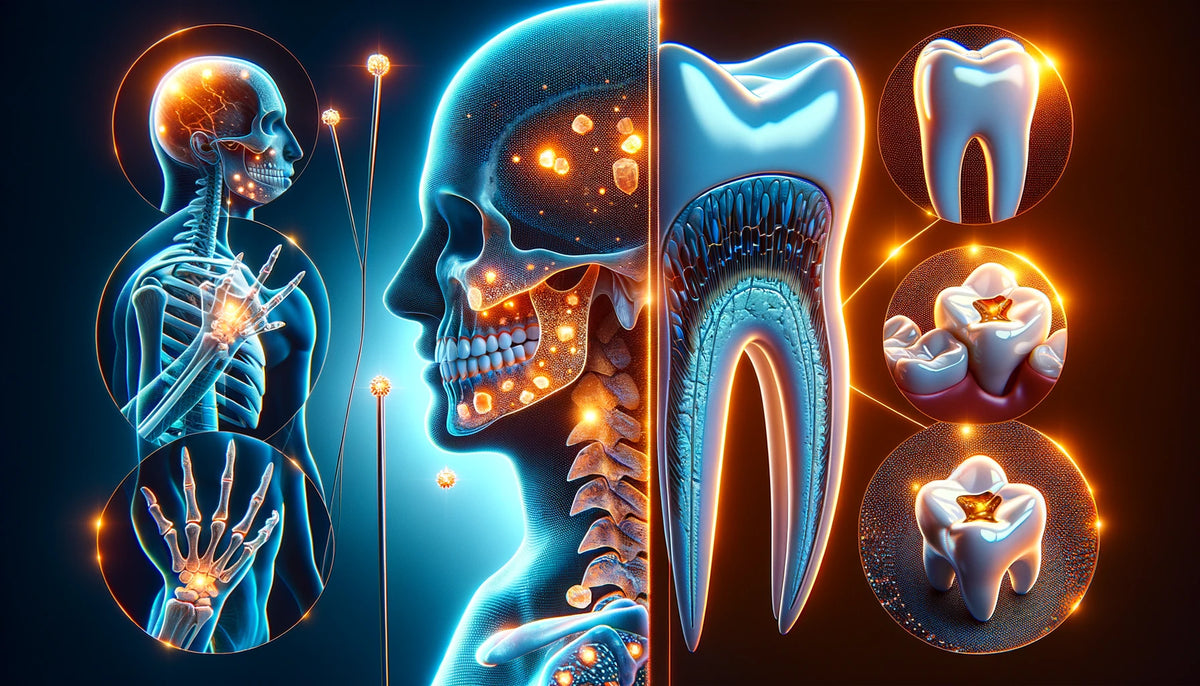
Hydroxyapatite, a naturally occurring mineral form of calcium apatite, is the principal inorganic component of tooth enamel and bone. Its formula is typically written as Ca_10(PO_4)_6(OH)_2, revealing that it comprises calcium and phosphate ions together with hydroxyl groups.
This bioactive and biocompatible material is incredibly important in the field of biomedicine due to its chemical and structural similarity to the mineralized tissues in human bodies. We find that hydroxyapatite plays a crucial role in a variety of medical applications, such as bone grafts and dental implants, improving the quality of life for many patients worldwide.

Our interest in hydroxyapatite extends beyond its medical applications. As researchers and scientists learn more about this material, it becomes apparent that it holds potential for future technological advancements. From enhancing the properties of biomaterials to its use in drug delivery systems, hydroxyapatite's unique characteristics make it an invaluable resource in advancing medical technologies.
Understanding hydroxyapatite is thus essential not only for those interested in the medical field but also for a broader audience who might benefit from its uses.
The ease at which hydroxyapatite bonds with natural bone, as detailed in studies on bone and hydroxyapatite ceramics, provides insight into its compatibility and effectiveness as an implant material.
We recognize the importance of this biological compatibility, as it ensures that the body readily accepts the material without adverse reactions, a crucial factor in the success of many medical procedures.
Hydroxyapatite, often recognized by its formula Ca₅(PO₄)₃(OH), is a naturally occurring mineral form of calcium apatite. The composition can be represented in various forms; one of which includes the hydroxide group: OH⁻. We assign great importance to its role in human biology, particularly in bone and tooth enamel, as it imparts strength and rigidity.
Principal Elements:
In its purest form, the stoichiometry of hydroxyapatite maintains a consistent 5:3 ratio of calcium to phosphate. However, natural and synthetic variations may exhibit substitutions in elemental composition.
Elemental Substitutions:
We find these substitutions interesting, as they influence material properties like solubility and bioactivity, impacting their suitability for biomedical applications such as bone grafts and dental implants.
Hydroxyapatite (HAp), known for its similarity to human bone mineral, is characterized by a complex crystal structure and significant biocompatibility, rendering it highly suitable for biomedical applications. Our focus will be on its crystallography and specific physical properties that contribute to its usefulness in various applications.
The crystal structure of hydroxyapatite is hexagonal with lattice parameters that are indicative of its stable and rigid structure. The precise arrangement offers distinct planes that are favorable for cell attachment and growth. It is this crystalline arrangement that imparts hydroxyapatite with its excellent mechanical stability and the ability to support bodily loads when implemented in bone grafts.
Hydroxyapatite's physical properties perform crucial roles in its application. Notably:
These properties, along with its ability to promote osteoconduction and to be thermally stable, allow us to use hydroxyapatite in a range of biomedical contexts, from coating on implants to bone grafting materials.

Hydroxyapatite is a naturally occurring mineral form of calcium apatite with the formula Ca₅(PO₄)₃(OH), which we commonly find in our bones and teeth. It is crucial for providing strength and rigidity to our skeletal structure.
Hydroxyapatite is vital to both areas as it supports our body structure, allows us to accomplish daily functions, and is pivotal in the mineralization process during bone growth or repair.
Biocompatibility:
Medical Applications:

In our exploration of hydroxyapatite (HAp), we focus on its synthesis methods, each offering a unique approach to creating this important biomaterial with tailored properties for various applications.
We understand that wet chemical precipitation is a fundamental approach to synthesize hydroxyapatite. This method involves mixing aqueous solutions of calcium and phosphate precursors, typically under controlled pH and temperature conditions, to precipitate HAp. Due to its simplicity and cost-effectiveness, it is a widely used technique, allowing for the synthesis of hydroxyapatite with diverse structures.
Hydrothermal synthesis, on the other hand, employs high-pressure and high-temperature conditions in a sealed environment, such as an autoclave. We use this method to produce highly crystalline and pure hydroxyapatite. By adjusting the parameters, we can influence the morphology and size of the HAp crystals, as documented in studies that have synthesized needle-shaped hydroxyapatite through this method.
Lastly, we employ the sol-gel process for its versatility in creating hydroxyapatite with a high level of purity and homogeneity. This method involves the transition from a colloidal solution (sol) to an integrated network (gel), enabling us to control the porosity and composition, which is especially beneficial for applications in drug delivery and tissue engineering.

Hydroxyapatite (HA) has become a material of significant interest due to its biological compatibility and structural similarity to human bone and teeth. It is leveraged widely across various industrial domains, specifically in dentistry, orthopedics, and as a material used in catalyst and filtration technologies.
In dentistry, we find hydroxyapatite to be a critical element in the development of biomimetic coatings for dental implants and as a remineralizing agent in oral care products. It is well-established in toothpaste and mouthwash formulations for its ability to repair and prevent tooth decay by contributing to the natural remineralization process.
In the field of orthopedics, hydroxyapatite serves as a preferred material for creating synthetic bones and coatings for metallic orthopedic implants. Its compatibility with the human body allows it to facilitate the growth of new bone, integrating effectively with the surrounding tissue. The utilization of porous HA ceramics, in particular, is notable for their ability to promote bone ingrowth due to their structure which mimics natural bone.
Finally, as a catalyst, hydroxyapatite is seen in the chemical industry for a range of applications, such as biodiesel production and organic transformations. Its role in filtration processes on both laboratorial and industrial scales is crucial, providing a medium for vaccine production and virus attenuation.

In the realm of biomaterials, we focus on enhancing the properties of hydroxyapatite (HA) to bolster its application. Through modification and functionalization, we tailor HA to meet specific biomedical needs, such as drug delivery or improved integration with natural bone tissue.
We enhance the biological and mechanical properties of hydroxyapatite by doping it with different ions. Doping can improve HA's bone cell stimulation and antibacterial effects.
For example, the incorporation of silver ions into HA nanoparticles has shown to impart antibacterial properties, which is advantageous for use in bone grafts and coatings for implants. Similarly, substitution with strontium ions can lead to osteoinductive effects, promoting bone growth and healing.
We often combine hydroxyapatite with other materials to create composites that possess a blend of desirable characteristics.
For instance, the integration of HA into polymers can result in enhanced mechanical strength and bioactivity.
A notable development is the creation of hydroxyapatite composites with polyethylene glycol (PEG), which enhance biocompatibility and drug delivery capabilities. These composites are engineered to target specific tissues, making them highly effective in treating diseases or repairing tissue.

To understand the composition and properties of hydroxyapatite, we employ several analytical techniques. Each method provides us with unique insights into the material's structure and behavior.
We utilize X-ray diffraction (XRD) to determine the crystalline structure of hydroxyapatite.
XRD analysis reveals the purity and phase composition, which are critical for assessing the material's quality and suitability for various applications.
For example, a study on the synthesis and characterization of hydroxyapatite crystals showcases the use of XRD to identify the crystalline phases present in the samples.
In our assessment of hydroxyapatite, we also apply spectroscopy techniques, including Raman and infrared (IR) spectroscopy.
These methods allow us to probe the molecular vibrations and chemical bonds within the hydroxyapatite structure.
Investigations such as the biomimetic formation of hydroxyapatite often incorporate these spectroscopy methods to analyze the chemical substitutions in the crystal lattice.
Finally, we examine hydroxyapatite at the microscale using microscopy techniques like scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
These tools enable us to visualize the morphology and detailed nanostructure of the hydroxyapatite particles.
Insights into these characteristics can be found in research on the synthesis and characterization of various forms of hydroxyapatite, where electron microscopy forms a cornerstone of the analytical approach.

When we explore the environmental impact of hydroxyapatite, we primarily consider its role in pollution control and sustainable production methods.
Hydroxyapatite (HAP) is celebrated for its multifunctional properties, particularly in combating air, water, and soil pollution. This calcium phosphate compound is valued for its ability to remove contaminants such as heavy metals and radioactive nuclides, making it an alle
We are also keenly aware of the sustainability aspect of HAP production.
Our efforts in producing hydroxyapatite from biogenic sources or waste streams embody a circular economy approach.
For instance, the synthesis of HAP from food waste using wet precipitation methods not only reduces waste but also provides a sustainable route for creating valuable materials— approximately 1.5 g of HAP powder from such sources can be produced effectively, demonstrating a resource-efficient lifecycle.
In biomedical applications, we see constant innovation in creating safer and sustainable-by-design HAP nanomaterials.
Assessments of environmental hazards are integral to these processes, ensuring the responsible development of HAP with minimal ecological footprint.
| Attribute | Impact |
|---|---|
| Pollution control | Positive |
| Heavy metal removal | High Efficiency |
| Radioactive nuclide sequestration | High Efficiency |
| Sustainable synthesis | Food waste to HAP |
| Biomaterials application | Safer design with environmental assessment |

In this section, we'll tackle some of the most common inquiries regarding hydroxyapatite, a mineral integral to both healthcare and technological applications.
Hydroxyapatite is a crucial component of bone tissue, providing rigidity and strength. Our bones are essentially a composite material with a collagen matrix reinforced by hydroxyapatite crystals, which accounts for their impressive structural integrity.
In dental care, hydroxyapatite is valued for its compatibility with natural tooth enamel.
It's used in restorative materials and toothpastes to promote remineralization and repair of the tooth surface, helping to prevent decay and maintain oral health.
Synthetic hydroxyapatite is often produced from calcium phosphate.
This mimics the naturally occurring mineral found in our bones and teeth, ensuring biocompatibility when used in medical and dental applications.
Hydroxyapatite has a wide range of applications, including as a coating for orthopedic implants to enhance bone integration, in chromatography for protein purification, and in cosmetics for its adherence and safety profiles.
Hydroxyapatite contributes to oral health by its incorporation in toothpaste that binds to and helps rebuild the teeth's enamel. This can lead to enhanced resistance to tooth decay and improvement in the overall health of teeth and gums.
Hydroxyapatite has many benefits, but its use in medical treatments can sometimes lead to complications. These include infection or implant loosening.
Thorough research and clinical testing are important to navigate these potential issues.